Highlights
- Nitrate levels in Nebraska groundwater sourced for drinking are rising
- Water managers and others must understand the nuances of groundwater nitrate to effectively address its implications
- Water extraction volume has implications on observed contamination levels
Why study nitrate trends?
Nitrate contamination in drinking water is fast becoming a significant public health issue. This is because we now have evidence showing stronger relationships between nitrate levels in drinking water and adverse health outcomes. The Environmental Protection Agency (EPA) in 1992 set a standard of 10 mg/L (milligrams per liter) as the maximum amount of nitrate that is safe for people to consume. While the maximum contamination level (MCL) was set after compelling evidence suggested that high-nitrate levels caused methemoglobinemia (also known as “blue-baby-syndrome”), newer evidence of other health concerns are now emerging. Since the MCL was set in 1992, all community water systems are required to show that the water they distribute to their clients are below the MCL1 . Gaining a better understanding of nitrogen contamination trends over time will help decision-makers better prepare for current and future drinking water concerns. Nitrate trends can show which areas or water systems will be approaching the MCL faster than others. Such information will aid in targeting mitigation efforts by water managers and policy makers.
This study is focused on public water systems and their trends in nitrate contamination levels. Statewide, the analysis shows that nitrate levels have been increasing over the years. Although this increase does not seem alarming overall, we must realize that it is an average, which disguises the highest and lowest contamination levels. Our intent is to further our research to discover more accurate assessments of nitrate level trends in Nebraska.
What are trends?
Trends are often used to understand the movement of contaminant levels over time. They can inform how fast (or slow) contamination levels have risen (or fallen) over the years and can show evidence of human activities in the state that may have contributed to nitrate leaching. These trends can also verify attributable health findings (such as cancers, adverse birth outcomes and methemoglobinemia, etc.) by identifying the association between nitrate contamination over the years and incidence of unfavorable health outcomes in the same time periods.
Examining trends involves researching the variance of average nitrate levels over time, divorced of aspects in wells or geographies that may systematically increase or decrease nitrate observations. For example, wells that are poorly constructed may show higher nitrate levels in water samples, while robustly constructed wells may show lower nitrate levels. Whether wells are in sandy soil or clay soil can also affect nitrate observations in water. This project looks at the state average nitrate level without construction or locational aspects influencing the contamination levels over time.
Some background
According to the United States Geological Survey (USGS), steady increases in nitrate levels in drinking water can be attributed to human activities. The USGS determined that naturally-occurring levels of nitrate in groundwater are below 2 parts per million (ppm). Beyond this level naturally occurring in groundwater, nitrate contamination of groundwater has several human sources. In more populated areas, contamination occurs due to leaching from septic systems. In agricultural areas, nitrate leaching occurs from fertilizer applications and farm animal manure. Nebraska, as a leading agricultural state in both row-crop agriculture and animal production, may be more vulnerable than other comparable states to the effects of nitrate leaching. This vulnerability may be exacerbated in parts of the state that are characterized by well-drained soils and shallower groundwater systems that facilitate nitrate migration from soil into groundwater. This, coupled with the fact that most drinking water needs in Nebraska are met from groundwater sources, also means that any contaminants leached into the water table will cycle back into the drinking water supply sooner than other comparable locations.
Nebraska’s nitrate trends
Despite all “public wells” being required to report nitrate levels, only “community wells” distribute water for household needs on a regular basis and are almost always situated in Wellhead Protection Areas (WPAs).2 Other wells that are considered “public wells” but not “community wells” are called “non-community and non-transient” and “non-community and transient” wells.
On average, between 1995 and 2022, wells situated in WPAs show a slightly larger increase in average nitrate levels compared to the public well average (see Figure 1). Figure 1 also shows that the average nitrate concentration in public wells is almost entirely driven by nitrate levels in community wells (i.e. the average public well nitrate trend follows that of wells in WPAs). Though the nitrate level data implies the WPAs are not making a difference, community wells extract a higher volume of water at any given time compared to other public wells, resulting in more contaminants being observed over time. Overall, Figure 1 shows that nitrate trends may not be showcasing the true complexity of the problem in Nebraska, which clearly warrants further investigation.
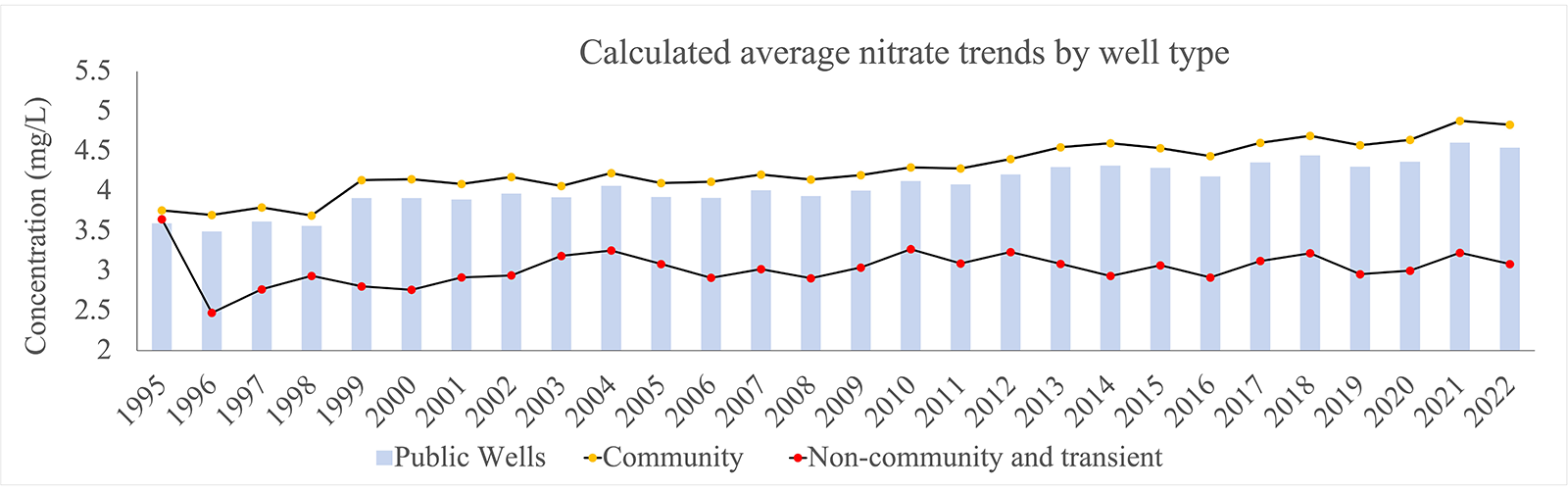
Source: Nebraska Groundwater Quality Clearinghouse
The delicate dance of managing safe drinking water
Public water systems are, on average, below the EPA standard of 10mg/L. While this might be comforting to many, maintaining contamination at safer levels is not achieved without effort. These efforts include retiring wells that are driving the contaminant average higher, drilling new wells and redrilling existing shallower wells, among other nitrate mitigation techniques. Figure 2 shows nitrate levels by well type, comparing wells that were likely retired between 1995-20043 , continued operations from 1995-2022 and likely added after 20044 . This combined figure shows that high nitrate wells were retired and low nitrate wells were added to the system of public wells to keep the average level of nitrate contamination lower. However, what is concerning to note is that all wells, regardless of when they were added and their initial nitrate concentration, have the same progressive increase in nitrate levels. This, again, can be attributed to the volume of water that is being extracted5 , pulling contaminants more quickly through the aquifer.
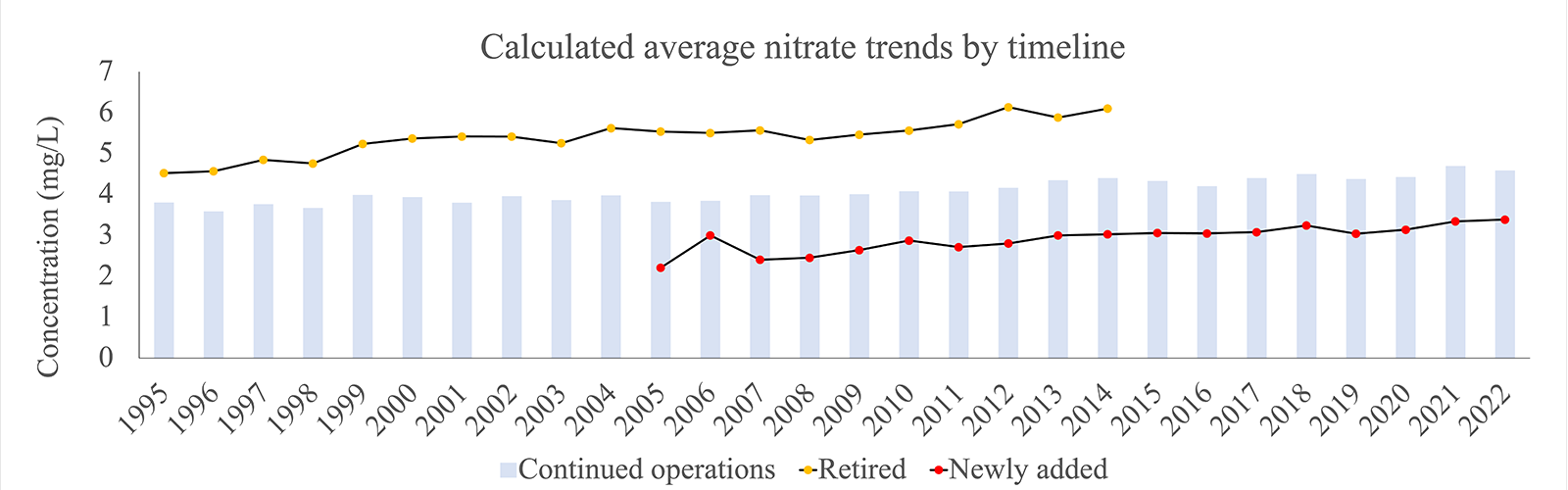
Source: Nebraska Groundwater Quality Clearinghouse
Above average nitrate levels
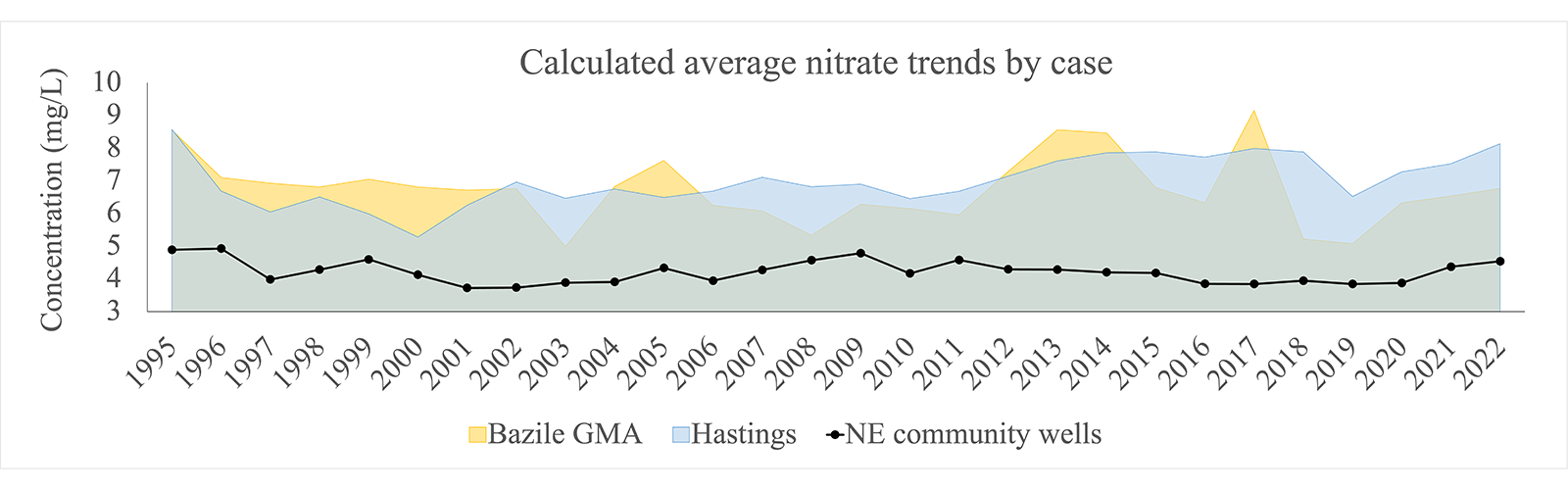
While the current average level of contamination in Nebraska community water systems is not approaching the MCL, there are many specific areas in the state that are approaching the MCL. These areas are highlighted in Figure 3: WPAs in Bazile Groundwater Management Area (BGMA) and the City of Hastings. Both cases show their individual community water systems are much closer to the MCL (hovering around 7mg/L) compared to the average community water system in Nebraska. In both cases, water managers have been actively trying to control quality in source water used for drinking. The nitrate level data suggests that managing water quality after contamination may not be as effective as reducing the contaminants going into the groundwater in the first place.
The crux of the issue
The nitrate trends analysis suggests that, in general, nitrate levels in groundwater used for drinking is increasing. This increase is despite consistent efforts to manage water quality, which are not only time-consuming, but also expensive. The main take-away from this report is that nitrate management is complex and nuanced. Some parts of Nebraska that have been grappling with nitrate contamination in drinking water will continue to do so. These areas may have to purify their drinking water before the rest of the state does.
However, water purification comes with its own set of complications and costs. Nitrate filtration involves specialized systems, such as reverse osmosis (RO) or ion exchange (IX), which are more expensive than traditional carbon filters. Additionally, using these filtration systems means more water needs to be extracted to serve the same demand, because RO (or IX) systems require at least 15% more water as inputs to produce the current demand for water . Even if pumping more water is not a significant hardship, there is also the issue of wastewater disposal to consider. There are still many variables and “what-if” scenarios for local governance to examine when developing solutions for nitrate prevention and mitigation.
Acknowledgements
This work was supported by the Water, Climate and Health Program, UNMC College of Public Health project, Health and Economic Impact Analysis of Nitrate Contamination of Groundwater in Nebraska, and the Daugherty Water for Food Global Institute at the University of Nebraska.
This work would not have been possible without the input and guidance received during many conversations with personnel from the Nebraska Department of Environment and Energy, Hastings Utilities, The Bazile Groundwater Management Area Project, as well as researchers from the University of Nebraska System, especially Crystal Powers (Daugherty Water for Food Global Institute at the University of Nebraska, Nebraska Water Center, and Nebraska Extension), Katie Pekarek (Nebraska Extension) and Renata Rimšaitė (Daugherty Water for Food Global Institute at the University of Nebraska). Appreciations to the Nebraska Department of Environment and Energy for providing access to the Nebraska Groundwater Quality Clearinghouse data that made this assessment possible. The views expressed in this report are my own.
Supplementary section: technical notes
The objective of this research is to describe the trajectory of nitrate contamination in drinking water in Nebraska. Data used for the following analysis is from the Nebraska Groundwater Quality Clearinghouse (NGQC). The NGQC database consists of groundwater quality data collected via state and local agencies and spans across several decades (from 1969 onward). This database, therefore, allows for consistent estimates of average nitrate levels.
Nitrate observations in groundwater depend on many determinants. Some of these determinants are specific to geology/geography such as elevation, soil type, groundwater gradient and land- use. Other determinants, such as screen placement, well casing and well depth are linked to well construction. Therefore, it is important to account for these determinants when estimating average trends, which can be achieved by adding variables that describe each determinant into the statistical model or by using alternative model specifications. However, this was not possible in this analysis due to data constraints. Instead, this issue was studied using well-level fixed effects that absorb any differential effects by individual wells that are observable and unobservable. Although most nitrate determinants are time invariant, there are seasonal variations, which was accounted for using interactions between well-level fixed effects and month fixed effects. This captures average monthly nitrate levels over the years by individual facility (well). This accounts for months that are irrigated, months that no agriculture activities are conducted. By making these allowances the variables of interest (average annual nitrate levels) remain independent of any seasonal effects that might consistently show-up across the years. This model is described in the following equation.
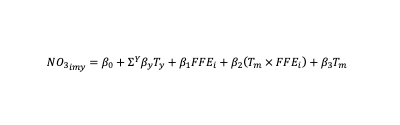
Where, NO3it is the measure of nitrate observation per facility (FFE) identified by i at year y and month m. The coefficients of interest here are βy which are our trends estimates that indicate the annual averages of nitrate observations for a given administrative unit. Post-estimation, we predict the annual average nitrate levels per year to depict temporal trends.
1 There is no such standard for private wells.
2 The Wellhead Protection Program (WPP) is a voluntary program that local communities could use to protect their water supplies with the assistance of the Nebraska Department of Environment and Energy (NDEE). WPAs are, therefore, areas that wellheads are situated.
3Based on wells not appearing in the sample after 2014
4These wells first appeared in the sample after 2005 and remained until 2022
5Engineering research has the evidence that shows how contaminant levels increase (exponentially) with the volume of water that is extracted.
6Larger RO/IX systems are about 85% more efficient than point-of-use systems that might need more than twice as much water inputs.


